What Monomer Should Be Used to Make the Following Polymer:
In order to form a condensation polymer from teh following monomer which other monomer should be selected. The compound 6-aminohexanoic acid is used to make the condensation polymer nylon-6.

Solved 2 Draw A Structural Formula Of The Monomer Of The Chegg Com
3 pts 1 CH2CHCH2CH3 II CH3CHCHCH3 III CH2CHCH3 IV CH2C CH32 18 1 6.
. Ethyl methacrylate and odorless monomer _____ is the industry standard monomer liquid. The polymer formed from the monomer CH2CH-CN is-CH2CH-n I CN. Memorize flashcards and build a practice test to quiz yourself before your exam.
Wash and scrub hands and nails d. 78 79 These monomers can be classified in two main categories. A solid amorphous polymer precipitates out of the saturated solution.
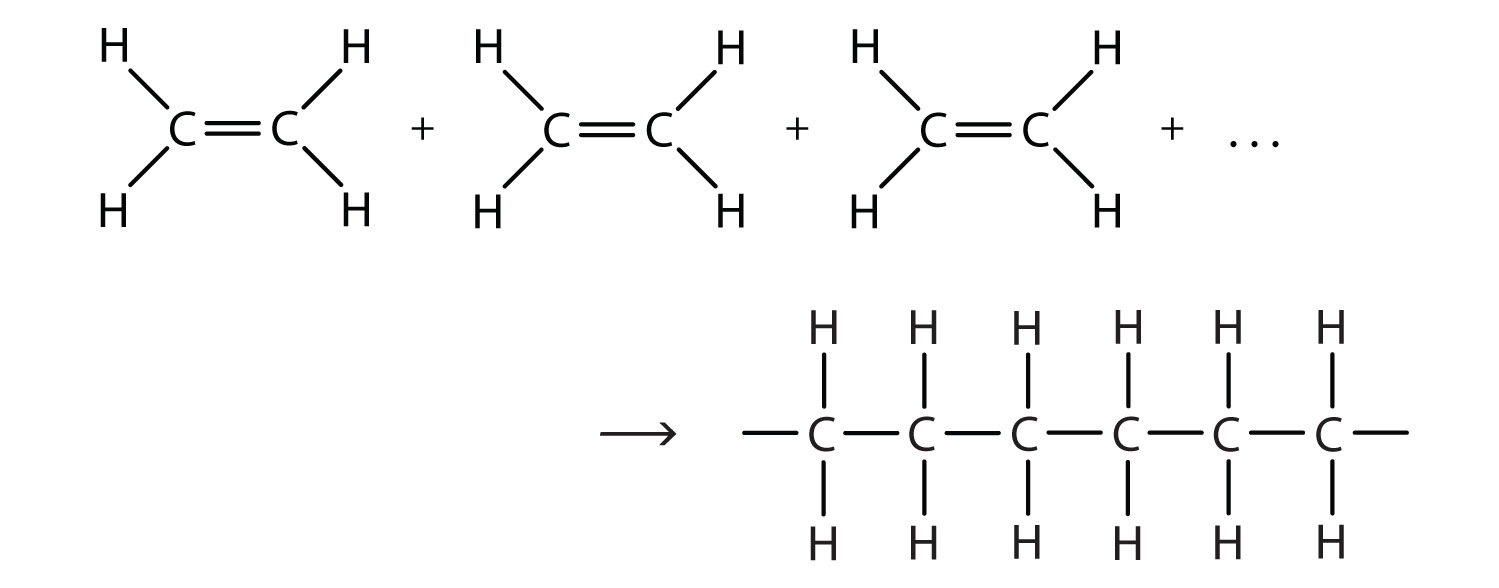
Polymerization is not always successful however because of a competing side reaction. The monomers are unsaturated compounds. Also asked what is the monomer used to make Polyethene.
Given the relative rate of hydrogen abstraction for a radical bromination mechanism as discussed in class follows the order of. Classification Based on Synthesis. A monomer is a molecule that can be polymerized or joined with other molecules to form a macromolecule.
Monomers bond together to form polymers during a chemical reaction called polymerization as the molecules link together by sharing electrons. The temperature and pressure at which the polymerization occurs. Start studying the Chapter 17.
The functional group found in proteins is called a an. Protein is obtained as a result of polymerization of monomer o-amino acids. 1 vinyl monomers for which the reactive end group is a carbocation and 2 heterocyclic monomers bearing one or two heteroatoms within the ring structure for which the reactive end group is an onium ion eg.
Although this is the usual way to draw. Synthetic polymers are man made polymers. When liquid monomer in solution is converted into an insoluble polymer the following possibilities arise.
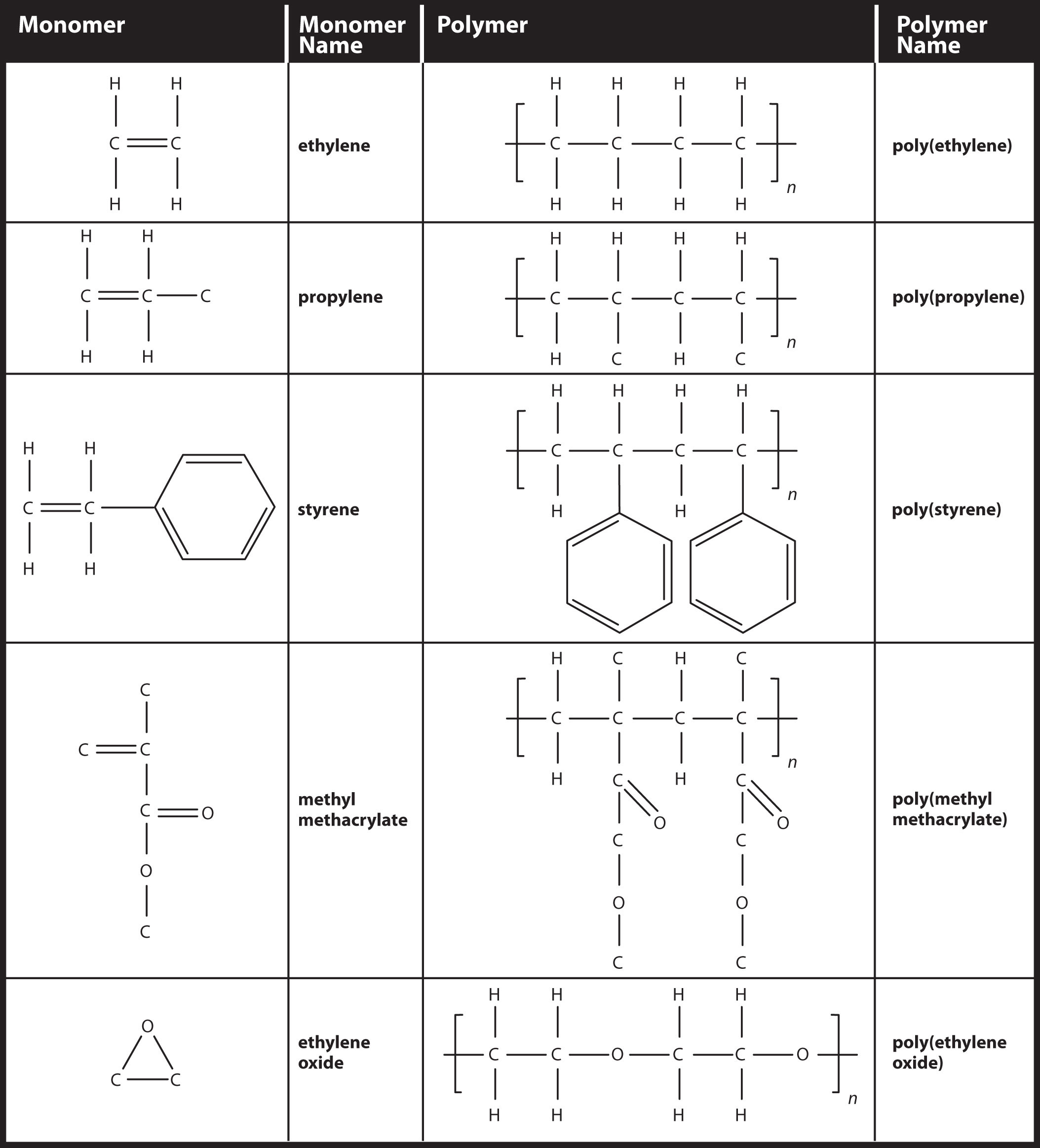
The diisocyanate most used is 24-diisocyano-1-methylbenzene and the diol can be a polyether or a polyester with hydroxyl. Polyesters plastic nylon and proteins all have monomers as their building blocks. B The monomers used to make polymers are essentially universal.
What one monomer was used to prepare the polymer below. H H I I H-O-C-C-O-H I I H H. Heteroatoms greatly alter the chemical and physical properties of a hydrocarbon.
In contrast 13-cyclohexadiene reacts more slowly and 13-cycloheptadiene is practically unreactive. If this happens which of the following should you follow. Cyclopentadiene reacts very rapidly in Diels-Alder reactions.
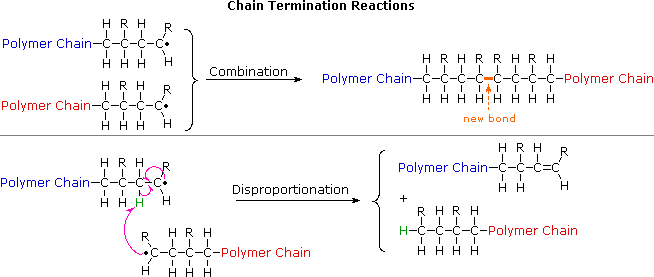
E Monomers are joined together by the process of hydrolysis. Mark all that apply. A monomer is covalently joined to a growing polymer chain by.
The monomers combine with each other via covalent bonds to form larger molecules known as polymers. For example polythene polystyrene PVC nylon and dacron. Use a hand sanitizer.
B solid crystalline polymer precipitates from the saturated solution. Monomers are smaller molecules and when bonded together make up polymers. A Cells typically make all of their macromolecules from a set of 40-50 common monomers and a few other ingredients that are rare.
Monomers bearing electron-releasing groups are susceptible to cationic polymerization. In doing so monomers release water molecules as byproducts. 1 calculate the expected.
Monomer Liquid and Polymer Powder Nail Enhancements flashcards containing study terms like A _____ file or buffer is used to thin the enhancement product to prepare the enhancement for a refill or rebalance. Stanislaw Penczek Eric J. The longer the chain the higher the molecular weight.
Choose the correct explanation for this trend. In the process a water molecule is formed. The essential ingredients are a diisocyanate and a diol.
Monomers will combine to make polymers which are long chains of repeating units. Coarse-grit medium-grit fine-grit shiner The tacky layer. The way the polymer is collected which can produce either a more or less random alignment of the polymer chains or a fabric in which the chains are.
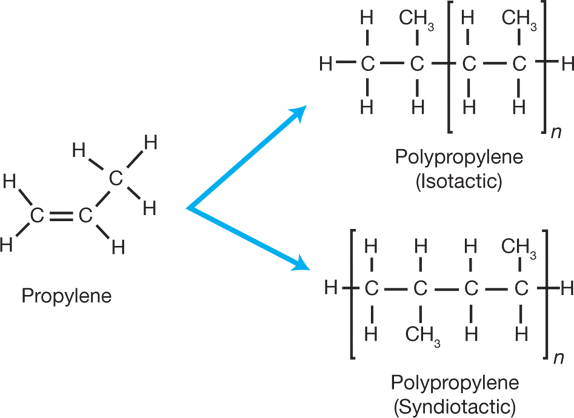
The monomers used to make other addition polymers are drawn in a similar shape to ethene for example propene. Goethals in Comprehensive Polymer Science and Supplements 1989. Instructions for using monomer liquid and polymer powders can vary from the text.
Ethylene C 2 H 4 is a stable molecule with two carbon atoms and a double bond. Ray O CN O 허 힝 하 Z z z O Identify the reactants you would use to prepare the following compound via a Diels-Alder reaction. H-N- hexagon-N-H I I H H.
The first step to prepping the nails before applying a nail coating should be to. The given polymer is obtained by the monomer of ethyl methacrylate. Polyethylene PE is a made by the reaction of multiple ethylene molecules in the presence of catalyst to break the double bond and connect the carbon atoms into a chain Figure 1.
CN CN Select the suitable reactants. CN NC 0 NC CN ONC CN. The solvent in which the monomer is polymerized.
-Fatty acids are the monomers for lipids for example and regardless of how they are bonded as a saturated or unsaturated fat for example they will form lipids. Which step of addition polymerization will result to the addition of one monomer molecule to form a new polymer molecule which is one repeating unit longer with a new active center. -Nucleotides form nucleic acids eg.
Step 1 of 3. The very widely used polyurethane foams can be considered to be either block polymers or copolymers. Polymer powders come in a variety of colors.
100 3 ratings for this solution. D DNA is built from just four kinds of monomers. What monomer should be used to make the following polymer.
Block polymers also can be made easily by condensation reactions. Polyethylene is vinyl polymer made from the monomer ethylene. Remove shine with a mediumfine 240-grit abrasive or buffer b.
A monomer is a single atom small molecule or molecular fragment that when bonded together with identical and similar types of monomers form a larger macromolecule known as a polymer. 46323 Influence of phase separation. The identity and amount of the reagent used to crosslink the polymer chains.
What colors do monomer liquids and polymer powders come in. The most common type of monomers in use is the sugars glucose and fructose. What monomer should be used to make the following polymer.
Use a nail dehydrator c. Superglue is made from this monomer cyanoacrylate. Addition or chain polymers It involves the repeated addition of monomers to the polymer chain.
C Monomers serve as building blocks for polymers.
Comments
Post a Comment